The Kinetic Molecular Theory & Hot Air Balloons – Our Guide

The kinetic molecular theory is used to explain the behaviour of gases as a movement of a large number of molecules in energy.
This kinetic molecular theory can be used to explain the hot air balloons since kinetic energy inside of balloons helps them to lift when the balloon is being heated inside, this is because it becomes lighter and more buoyant.
In our guide below, we will take you through what the kinetic molecular theory is as well as the Charles law, how it relates to hot air balloons, the history of physics and hot air balloons as well as Chinese lantern theory explain kinetic energy.
What Is The Kinetic Molecular Theory?
Before we can understand how the kinetic molecular theory works with hot air balloons we need to understand exactly what this theory is first.
The kinetic molecular theory is used to explain gas properties and how gas works, we’ve listed the basic assumptions of this theory down below, then we will move on to how it relates to explaining gases.
- Gas particles move independently in a straight motion creating large spaces during particles and allowing them to be compressed.
- These particles can expand in volume and collide with each other as well as the walls around them.
- The faster the particles than the more kinetic energy that they possess.
- Increasing temperature increases the speed of particles which then increases kinetic energy.
What Is The Charles Law?
Now we know the main assumptions of the kinetic molecular theory we can use it to explain gas through the Charles law.
The Charles law states that at a consistent pressure, the gas will increase and decrease according to high and low temperatures, causing gas to rise.
How Does The Kinetic Molecular Theory Relate To Hot Air Balloons?
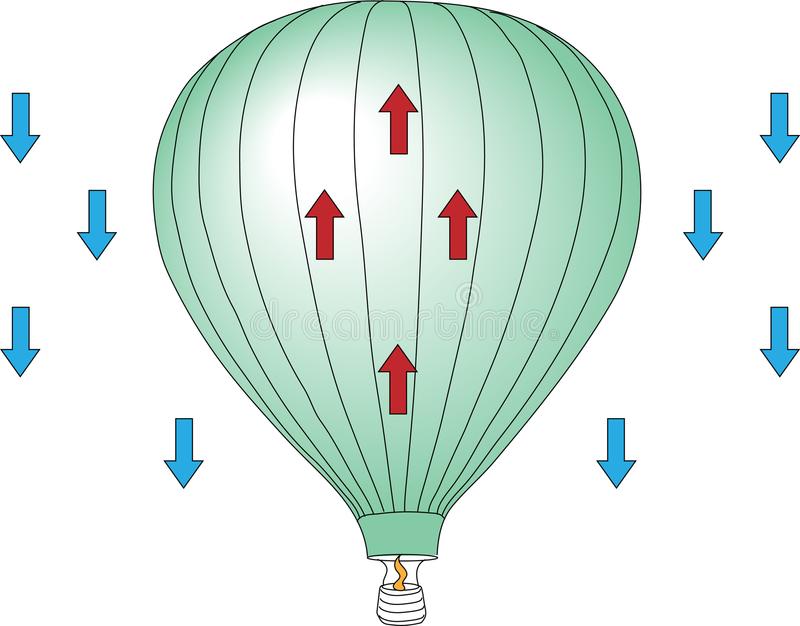
Now we know what the Charles law and kinetic molecular is we can relate it to hot air balloons and how they rise with kinetic energy inside.
- Stage one – As the air gets heated inside of the hot air balloon the kinetic energy inside increases since the particles are moving faster creating a lift to help the balloon move upwards and float, as you go upwards, the gas and air inside of the balloon needs to keep being reheated otherwise the volume of the gas inside will increase.
- Stage two – When the balloon starts to cool down the kinetic energy also decreases, meaning they move much slower. As the balloon contracts, the pressure inside remains balanced.
- Stage three – As returning to the ground the gas valve on the hot air balloon must be opened to allow the hot air to escape and take down the temperature inside of the balloon, as this happens gas molecules get decrease and the balloon will sink.
The History Of Physics & Kinetic Molecular Theory
If you want to understand how hot air balloons work, you need to be able to understand the history of physics behind them.
The first hot air balloons started in 1783 and used the cold air around the outside of the balloon to essentially float it since it has a higher density than the hot air on the inside. The propane gas burner at the base is heating the air inside and be changed in strength according to if you want to fly the balloon up or down.
At the top of a balloon, you will find a seal that can be opened and closed by the pilot to let hot air escape slow and steady.
Chinese Lanterns To Explain Kinetic Molecular Energy
One of the best ways to understand the kinetic molecular energy law and hot air balloons is by looking at Chinese lanterns which follow the same mechanism.
- Uniform temperature – If a Chinese lantern has no flame or a balloon then the outside and inside air molecules are the same temperature.
- Flame – When the flame is lit or the burner chemical energy is then transferred as kinetic energy to the air.
- Heating it – Now the hot air molecules spread up and out inside dispersing the cold molecules, these will be heated or lost.
- Temperature difference – As the hot air becomes hotter, the cold air is lost from the lantern since the volume is increasing in size.
- Density – A large expansion of hot air allows the balloon to rise since the air is less dense.
Frequently Asked Questions About Kinetic Molecular Theory & Hot Air Balloons
Can a hot air balloon sink?
Hot air balloons can sink if the gas valve at the top is left open for long enough, if the balloon gets a hole it could potentially sink too.
Are there different types of hot air balloons?
Yes, there are three main types of hot air balloons; Montgolfier which uses fire for lift, a hybrid balloon that operates the same way but has hydrogen gas on top and a pure gas balloon where hot air is not used, it is purely controlled by opening and closing the gas valve.
Which gas do hot air balloons use?
Most hot air balloons use propane as their gas, they can be mixed with other gases too so that fuel pressure can be maintained.
How do hot air balloons not catch fire?
Hot air balloons do not catch fire thanks to their nylon material which has a special fireproof coating.
Final Words
To conclude, the kinetic molecular theory relates to hot air balloons since it relates to how the temperature inside of the balloon rises. The higher the temperature of the gas molecules inside then the faster particles move, increasing kinetic energy, the lower the temperature becomes inside then the less kinetic energy.
I’m Annie, a twenty-something year old girl who loves hot air balloons. So much so, that I have a full time job as a Flight Instructor and it is all I love talking about. Something about being up there in the elevated altitudes helps all my stresses float away!
